Privo Technologies begins FDA Approved Clinical Trials
BOSTON, Oct. 24, 2018 /PRNewswire/ — Privo Technologies was granted the green light from the FDA and several hospitals this summer to begin patient recruitment for a prospective clinical trial (NCT03502148) targeting early-stage oral cavity squamous cell carcinoma (OCSCC).
Privo is now actively recruiting stage I and II OCSCC patients for this study.
About PRV111:
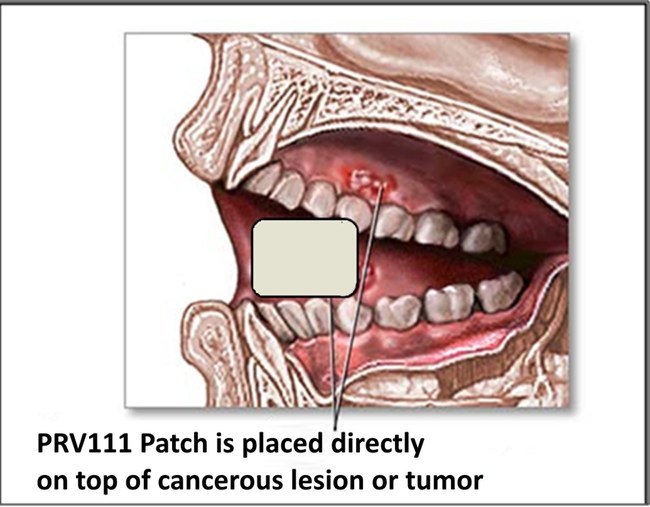
Privo’s PRV 111 (topical patch) is placed directly onto a mucosal tissue. This topical patch is intended for the locoregional treatment of oral cavity squamous cell carcinoma without systemic toxicity.
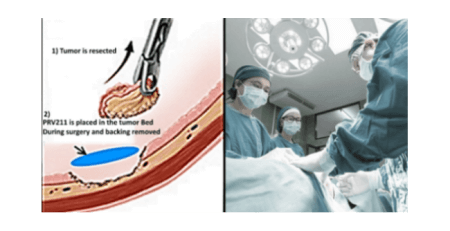
Privo has developed a nano-engineered product called PRV111 consisting of a topical patch designed to deliver and retain high concentrations of various existing systemic agents within the primary tumor and associated nodal basins.
Dr. Manijeh Goldberg, Privo’s founder, and CEO is very encouraged by the result of Privo’s preclinical studies and expects PRV111 to provide a safer and more effective treatment option to patients suffering from oral cancer.
When placed on the tumor, PRV111 releases and retains cisplatin-loaded particles into the tumor, resulting in a dramatic reduction of tumor size, without the accompanying systemic side effects of intravenous cisplatin (i.e., nephrotoxicity and neurotoxicity). Local and regional effects of PRV111 are expected to improve tumor resectability, decrease the need for post-operative radiation and chemotherapy and improve patient survival. According to the National Cancer Institute, 76% of all newly diagnosed oral cancers are locoregional (29% local and 47% regional), and the 5-year survival is about 65%. Privo aims to improve the survival rates with its PRV111 intensive topical treatment. This organ-sparing therapy can preserve oral cavity form and function while improving locoregional disease control, which is the primary driver of disease-specific and overall survival. In patients with the regional metastatic disease, PRV111 can be combined with the standard of care chemo-radiation regimens to provide improved locoregional control while maintaining a tolerable side effect profile and improving quality of life.
When asked about Privo’s clinical trial (NCT03502148), Dr. Vlad Sandulache, surgical oncologist and the clinical study’s principal investigator stated:
“We have learned much about the genomic, epigenetic and proteomic profile of oral cavity squamous cell carcinoma (OCSCC) over the last decade. Unfortunately, this knowledge has not translated into effective novel therapies or improved survival for our patients. Cisplatin, although an old drug, remains by far the most effective systemic agent available for patients with OCSCC; however systemic toxicity limits utilization and can prevent use in patients with pre-existing renal disease. PRV111 presents a unique opportunity to revolutionize utilization of this proven agent. Not only does locoregional delivery completely eliminate systemic toxicity, but the intra-tumoral cisplatin concentrations generated by PRV111exceed those achievable through intravenous administration by over an order of magnitude. This represents a qualitative leap in potential effectiveness which could overcome traditional mechanisms of cisplatin resistance previously described in OCSCC.”
Clinical Trial Info
For information regarding this trial, please visit https://clinicaltrials.gov/ct2/show/NCT03502148.
Participating hospitals include:
- Baylor Clinic, 6620 Main Street, Houston, Texas 77030
- Baylor St. Luke’s Medical Center, 6720 Bertner Avenue, Houston, Texas 77030
- Baylor College of Medicine, 1 Baylor Plaza, Houston, Texas 77030
- Houston Methodist Hospital, 6550 Fannin Street, Suite 1701, Houston, Texas 77030
- Ben Taub Hospital, 1504 Ben Taub Loop, Houston, Texas 77030
- Harris Health System – Smith Clinic, 2525A Holly Hall Street, Houston, Texas 77054
- Memorial Hermann-Texas Medical Center, Houston, Texas 77030
- The University of Cincinnati Cancer Institute, Head and Neck Cancer Center, 234 Goodman Street, Cincinnati, Ohio 45219
- West Chester Hospital, 7700 University Drive, West Chester, Ohio 45069
Privo Technologies:
Privo Technologies, Inc. is a privately funded company with roots in the world-renowned Langer Laboratory at the Massachusetts Institute of Technology (MIT) in Cambridge, Massachusetts, USA. Privo is developing a novel, nanotechnology-based drug delivery platform capable of local and systemic delivery of a constant, controlled therapeutic dose through the mucosa. This approach eliminates needles, avoids stomach acids, can significantly reduce the toxic side effects of drugs and allows for higher dosing where the drugs can be most effective – all of which improve patient outcomes and compliance.
Privo’s pioneering drug delivery and chemistry expertise enables the company to create drug candidates for its internal development programs and generates opportunities for additional partnerships and collaborative alliances.
SOURCE Privo Technologies, INC